Research
Skeletal cell metabolism during bone development and pathology
Bone Metastasis
Skeletal progenitor metabolism, bone repair
and tissue engineering
Vitamin D
We aim to characterize the metabolic requirements for skeletal cell function
in health and disease
Skeletal cell metabolism during bone development and pathology
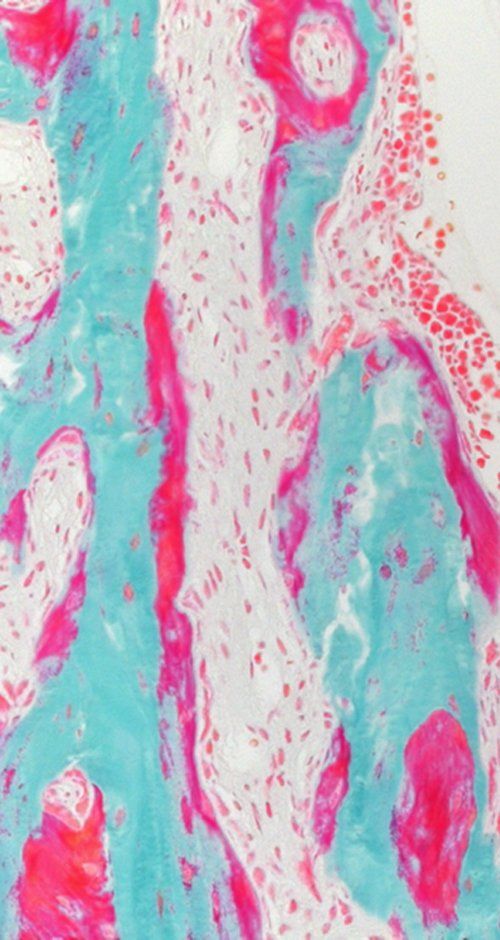
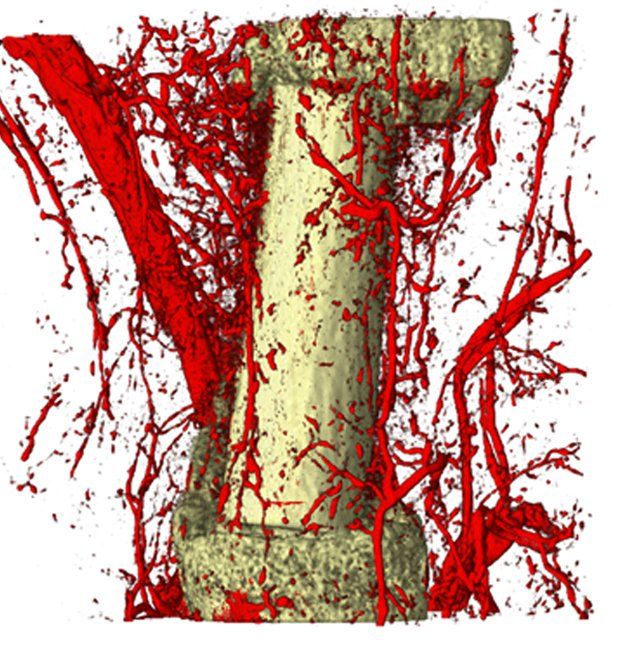
Bone development and its remodeling during adult life are highly anabolic processes requiring an adequate supply of oxygen and nutrients. Recent studies, including those from our lab, uncovered that skeletal cells have specific metabolic profiles that correspond to their specific nutritional microenvironment and regulate stage-specific functions. However, these metabolic programs are likely disturbed in skeletal pathologies such osteoporosis and osteoarthritis, but also in conditions of obesity and diabetes, which could contribute to bone cell dysfunction. More insight in nutrient use and metabolic pathway activity in different skeletal cell types will therefore not only help to better understand the mechanisms underlying bone development, but may also reveal novel metabolic targets to treat bone disease.
Bone Metastasis
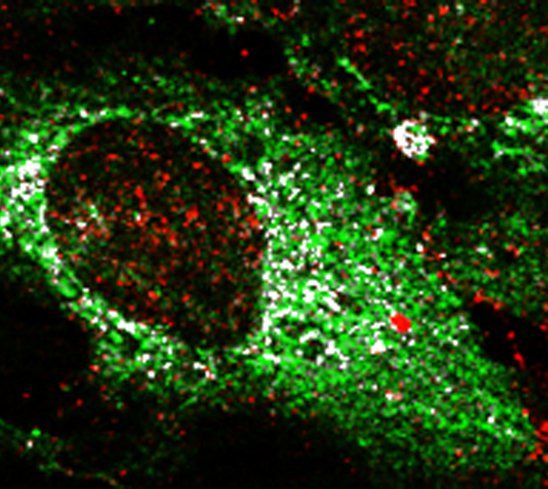
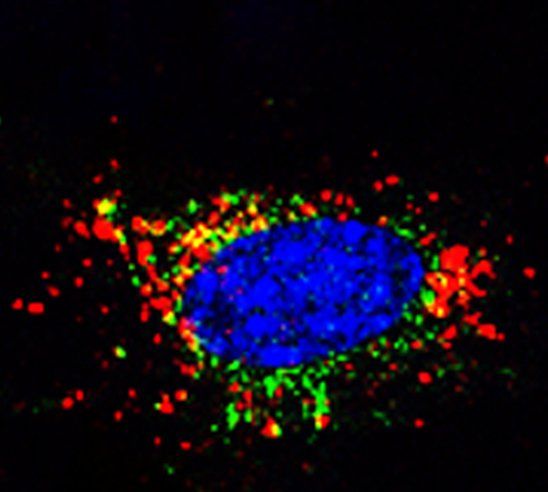
Bone is a metastatic predilection site for breast tumor cells and the loss of bone mass causes severe morbidity. To facilitate the development of disease-modifying therapies, insight in the early stages of bone metastasis is needed. Recent evidence shows that metabolic adaptations are not only important for tumor progression, but also for metastatic colonization of the lungs and brain. Tumor cells metabolically interact with cells in the local microenvironment and nutrient availability at the metastatic site will therefore influence the metabolic profile of tumor cells surviving and growing at the distant metastatic site. In the bone, metastasizing breast tumor cells localize close to osteoblasts and our aim is to characterize the metabolic profile of tumor cells that allows them to survive, grow and form osteolytic lesions.
Skeletal progenitor metabolism,
bone repair and tissue engineering
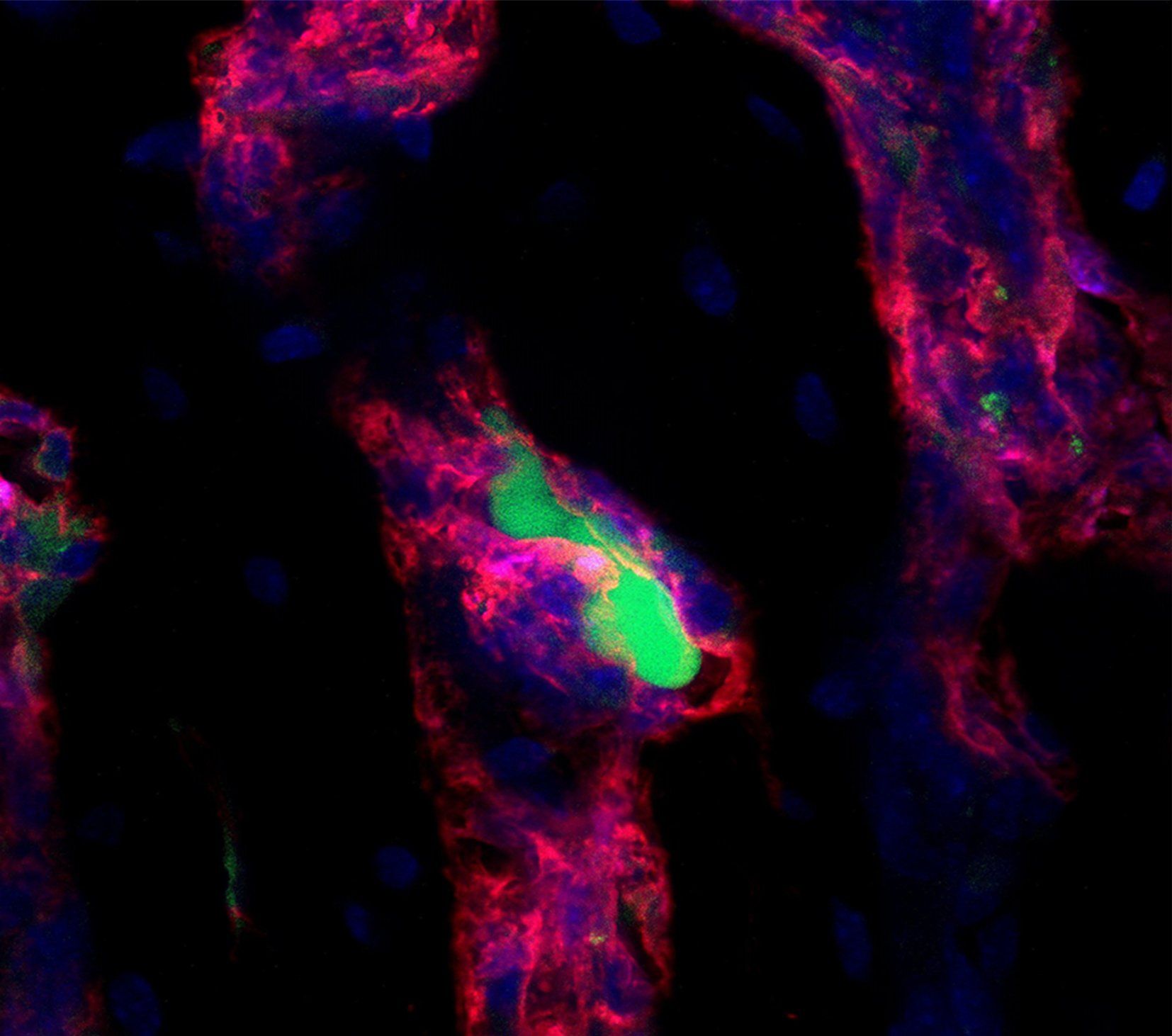
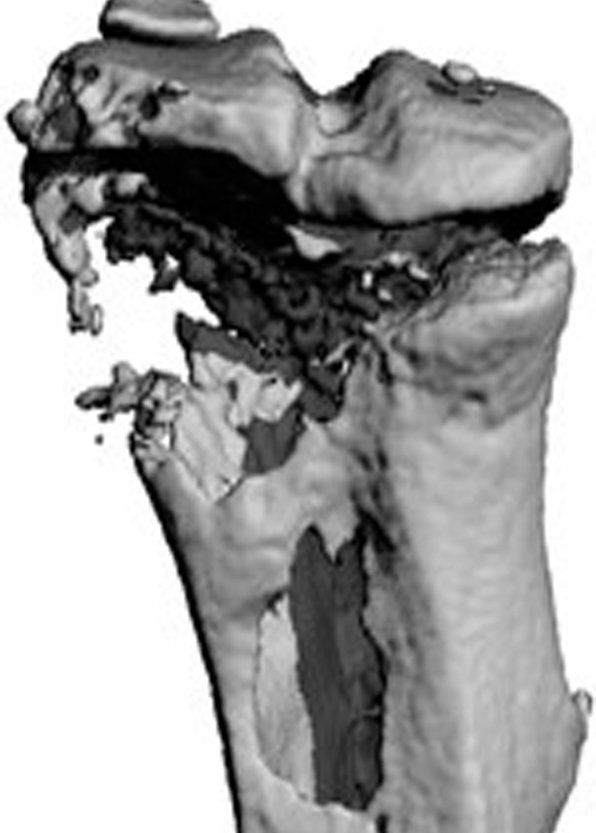
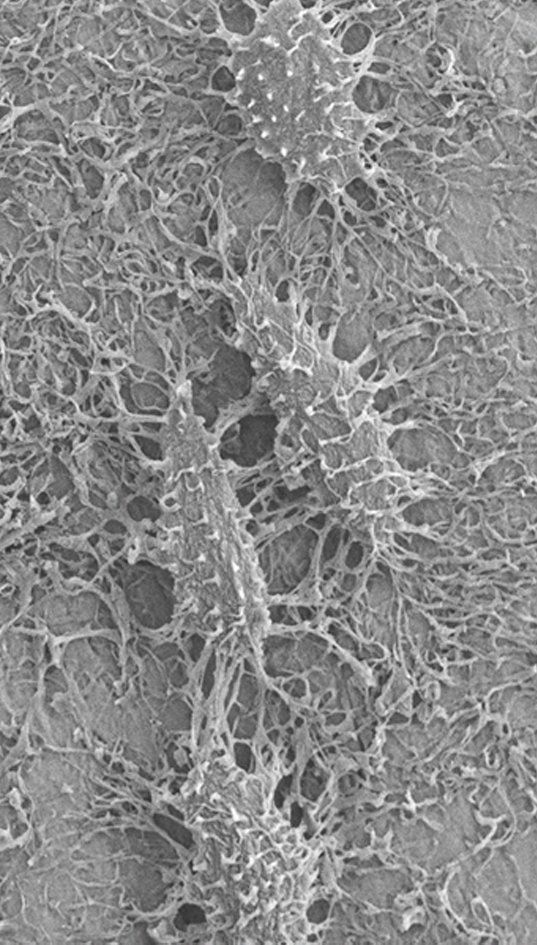
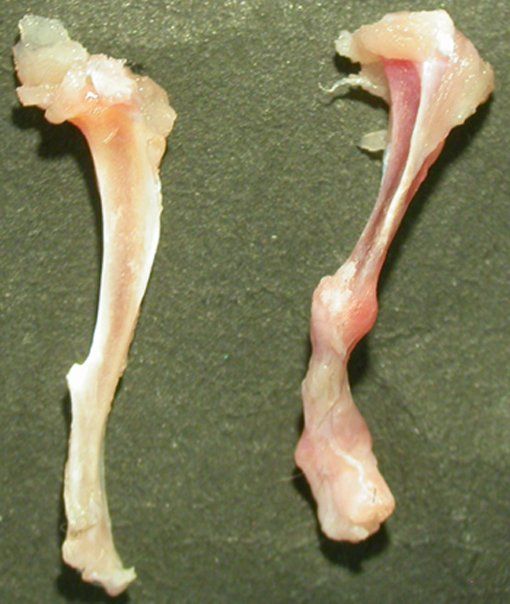
Small and uncomplicated bone fractures heal completely through intramembranous or endochondral bone formation. However, large or complex bone defects often do not heal properly, resulting in delayed or non-union. A promising strategy for these non-healing bone defects is bone tissue engineering, whereby skeletal progenitor cells seeded on a (growth factor loaded) scaffold are implanted in the bone defect. Still, this approach faces several challenges, since the regenerative potential of skeletal progenitors is lost upon expansion and the survival of the implanted cells is limited early after implantation. Indeed, the lack of blood vessels at the implantation site results in restricted supply of oxygen and nutrients to the implanted cells. We could show that hypoxia preconditioning of the skeletal progenitors improved their survival and the amount of bone formed (Cell Metabolism 2016). Our goals are to further understand which nutrients are necessary for cell survival in an avascular milieu and to investigate which metabolic pathways improve the preservation of skeletal stem and progenitors during
in vitro expansion.